Cs2AgBiBr6−xClx solid solutions – band gap engineering with halide double perovskites†
Abstract
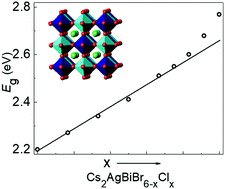
The halide double perovskites Cs2AgBiBr6 and Cs2AgBiCl6 form a complete solid solution. The cubic Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group symmetry and near complete rock salt ordering of Ag+ and Bi3+ are retained for all compositions, and the lattice parameter varies linearly between the two end-members. The band gap increases linearly between Cs2AgBiBr6 (Eg = 2.19 eV) and Cs2AgBiBrCl5 (Eg = 2.61 eV), but deviates upward from a Vegard's Law behavior once the chloride content exceeds ∼85%. The Cs2AgBiCl6 end-member has a band gap, Eg = 2.77 eV, that is 0.10 eV larger than the extrapolated value from a Vegard's Law fit. Analysis of the powder X-ray diffraction peak shapes shows that microstrain increases steadily as the Cl− content increases, before dropping precipitously for the Cs2AgBiCl6 end-member. The combined results give no evidence for long range ordering of halide ions.
m space group symmetry and near complete rock salt ordering of Ag+ and Bi3+ are retained for all compositions, and the lattice parameter varies linearly between the two end-members. The band gap increases linearly between Cs2AgBiBr6 (Eg = 2.19 eV) and Cs2AgBiBrCl5 (Eg = 2.61 eV), but deviates upward from a Vegard's Law behavior once the chloride content exceeds ∼85%. The Cs2AgBiCl6 end-member has a band gap, Eg = 2.77 eV, that is 0.10 eV larger than the extrapolated value from a Vegard's Law fit. Analysis of the powder X-ray diffraction peak shapes shows that microstrain increases steadily as the Cl− content increases, before dropping precipitously for the Cs2AgBiCl6 end-member. The combined results give no evidence for long range ordering of halide ions.


 Please wait while we load your content...
Please wait while we load your content...