Instantaneous detection of explosive and toxic nitroaromatic compounds via donor–acceptor complexation†
Abstract
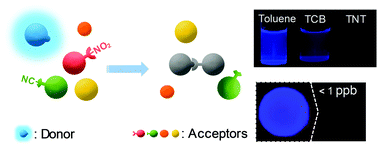
Designed carbazole-based novel fluorophores (TPCA0M and TPCA6M) exhibit a sensitive response to toxic and explosive nitroaromatic compounds (NACs) via complex formation, and their detection sensitivity or selectivity can be demonstrated by comparing their emission-quenching rate constant or the adoption of similar-structured aromatic compounds as a negative control. The results of time-resolved fluorescence spectroscopy indicate that the static quenching mechanism is involved in NAC detection. In addition, it has been demonstrated by UV-visible and 1H-NMR spectroscopy studies that the emission quenching of the devised fluorophores originated from complex formation with NACs. The difference in the detection sensitivity of the fluorophores for the corresponding NACs can be connected to both the different steric effects of the fluorophores and the degree of energy off-set between the fluorophores and the NACs. A porous membrane-type detection platform, fabricated with TPCA0M, was found to be effective even at a TNT concentration of 1 ppb within 5 s, which reflects both the instantaneous detection capability and the exceptional sensitivity of the designed fluorometric chemosensors.


 Please wait while we load your content...
Please wait while we load your content...