Room temperature discotic liquid crystalline triphenylene-pentaalkynylbenzene dyads as an emitter in blue OLEDs and their charge transfer complexes with ambipolar charge transport behaviour†
Abstract
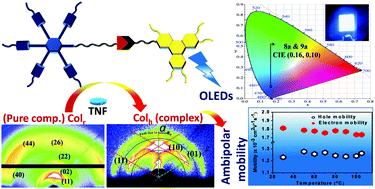
Six novel non-symmetric discotic liquid crystalline (DLC) dimers composed of pentaalkynylbenzene (PA) and triphenylene (TP) moieties were synthesized by using the CuI–Et3N catalyzed click reaction between terminal TP alkyne and PA azide. All the compounds exhibit mesomorphic behaviour at room temperature. Pure compounds 8a–b (n = 6, 8 & m = 1) and 9a–b (n = 6, 8 & m = 3) display a columnar centered rectangular (Colr) phase while 8c (n = 10, m = 1) and 9c (n = 10, m = 3) show a weak smectic (Sm) phase. Remarkably, the mesophase transforms from Colr and Sm to columnar hexagonal (Colh) on doping the pure compounds with electron-acceptor 2,4,7-trinitrofluorenone (TNF) molecules in a 2 : 1 TNF/compound ratio. The induction of stable Colh mesomorphism is driven through charge-transfer interactions of electron-rich PA and TP moieties with electron-acceptor TNF molecules. The thermal behaviour of the compounds was studied through polarised optical microscopy (POM), differential scanning calorimetry (DSC) and X-ray diffraction (XRD) studies. The performed grazing incidence small and wide angle X-ray scattering (GISAXS/GIWAXS) study showed good alignment ability of 9a in the Colr and complex 9a/TNF in the Colh phase. Additional information about the arrangement of molecules in the Colr and the Colh 2D lattice is deduced by the construction of electron density maps (EDM). The pure non-symmetric dimers 8a–c and 9a–c were blue luminescent in the solution state and shifted to green in the solid state because of aggregation. Hence, the fabrication of doped OLED devices by using 8a and 9a as an emitter with the configuration ITO/PEDOT:PSS/host:emitter/TPBi/LiF/Al illustrates the restoration of the electroluminescence (EL) spectrum to narrow and a blue shifted photoluminescence (PL) spectrum, which indicates the suppression of aggregates. Emitters 8a and 9a are doped with three different hosts: (carbazolyl)-1,1′-biphenyl (CBP), 4′,4′′-tri(N-carbazolyl)triphenylamine (TCTA) and 2,7-bis(carbazol-9-yl)-9,9-spirobifluorene (Spiro-2CBP), by varying the dopant concentration. The best EL performance is observed for the device fabricated with 8a (5 wt% in the CBP host) and 9a (3 wt% in the CBP host) with a maximum external quantum efficiency (EQE) of 2.1% and CIE coordinates of (0.16, 0.10). One of the studied complexes 8b/TNF exhibited an ambipolar charge carrier mobility of 1.78 × 10−3 cm2 V−1 s−1 and 1.25 × 10−3 cm2 V−1 s−1 for electrons and holes, respectively, at room temperature. The observed ambipolar mobility of the complex compound reveals its potential application as an organic semiconducting material.


 Please wait while we load your content...
Please wait while we load your content...