AIE-active difluoroboronated acylhydrozone dyes (BOAHY) emitting across the entire visible region and their photo-switching properties†
Abstract
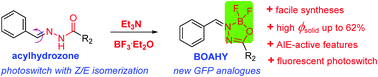
A new family of difluoroboronate anchored acylhydrozones, named BOAHY, have been developed as green fluorescent protein (GFP) chromophore analogues via a simple one-pot two-step reaction in moderate to good yields. They were well characterized by NMR, HRMS, X-ray crystallography and various spectroscopic techniques. These BOAHYs are weakly fluorescent in their solutions but emit brightly in the powder and film states across the entire visible region, with a maximum fluorescence quantum yield of 62%. They also exhibit notable aggregation-induced emission (AIE)-active features. Photoisomerization transformation under strong UV light irradiation at 365 nm as well as the viscosity-dependent fluorescence changes, indicate that the restriction of the intramolecular free rotation of the single bonds at the periphery is the main cause for the novel AIE process of these BOAHYs. In addition, the Z/E photoisomerization was studied for their potential application as photoswitching materials. UV light irradiation at 365 nm caused obvious fluorescence changes, and the photoisomerization was found to be reversible in a dark environment, which paves a new way for creating fluorescent photoswitches based on these planar conformations.


 Please wait while we load your content...
Please wait while we load your content...