Solid solutions of M2−2xIn2xS3 (M = Bi or Sb) by solventless thermolysis†
Abstract
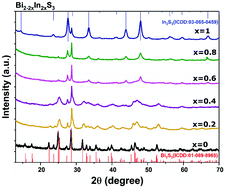
Tris(O-ethylxanthato)bismuth(III) [Bi(S2COEt)3], tris(O-ethylxanthato)antimony(III) [Sb(S2COEt)3] and tris(O-ethylxanthato)indium(III) [In(S2COEt)3] were synthesized and employed for the preparation of Bi–In–S and Sb–In–S solid solutions by solventless thermolysis. M2−2xIn2xS3 (where M = Bi or Sb) alloys were obtained using a mixture of In(S2COEt)3 and M(S2COEt)3 molecular precursors, with different mole fractions of indium x (0 ≤ x ≤ 1) at 300 °C. The structural, compositional, optical and morphological properties of the synthesized M2−2xIn2xS3 samples were characterized using a range of techniques including powder X-ray diffraction (p-XRD), scanning electron microscopy (SEM), energy dispersive X-ray (EDX) spectroscopy, Raman spectroscopy and UV-Vis absorption spectroscopy. The p-XRD data suggest that the incorporation of mole fractions of indium up to x ≤ 0.4 into M2S3 does not alter the orthorhombic crystal structure of M2S3. Higher quantities of indium (x ≥ 0.6) change the crystal structure to cubic M2S3. The elemental compositions from EDX data are in line with the stoichiometric ratios expected. SEM images reveal that the morphology of the M2−2xIn2xS3 (0 ≤ x ≤ 1) samples varies significantly with the changes in the indium mole fraction in the precursor mixture. Elemental mapping of the mixed samples M2−2xIn2xS3 (0 ≤ x ≤ 1) shows uniform elemental distributions of M, In and S in every sample investigated. The estimated band gap energies of Bi2−2xIn2xS3 films varies from 1.66 to 2.39 eV, while the band gap energies of Sb2−2xIn2xS3 films are in the range of 2.19–2.9 eV, and in both cases the energy can be tuned by variation of the indium content.


 Please wait while we load your content...
Please wait while we load your content...