Ambipolar transistors based on chloro-substituted tetraphenylpentacene†
Abstract
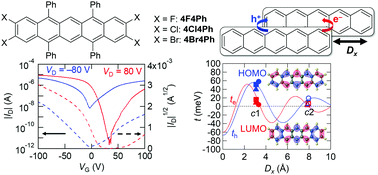
Thin-film transistors of halogen-substituted tetraphenylpentacenes are investigated. These compounds exhibit mainly hole transport, but the chlorine compound shows considerably higher performance than the fluorine and bromine compounds. In addition, the chlorine compound shows ambipolar properties, though the hole mobility is four times larger than the electron mobility. These compounds have basically the same crystal structures, but the remarkable halogen dependence is explained by the critical location of the LUMO levels, as well as intermolecular transfers, which sensitively change depending on the stacking geometry. In particular, hole and electron transfer exhibit different periodicity depending on the slip distance along the molecular long axis, and this is related to the appearance of the electron transport properties.


 Please wait while we load your content...
Please wait while we load your content...