Emissions from a triphenylamine–benzothiadiazole–monocarbaborane triad and its applications as a fluorescent chemosensor and a white OLED component†
Abstract
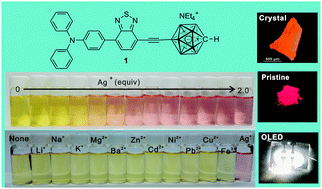
A fluorescent D–A–D′ triad (1), composed of monocarbaborane, benzothiadiazole and triphenylamine, was synthesized. By replacing monocarbaborane with phenyl (Ph), p-methoxyphenyl (PMP) or 4-pyridinyl (Py), three analogs (2, 3, and 4) were prepared for comparison. Monocarbaborane is an electron donating group based on the comparative analysis of NMR spectra and the systematic investigation of their photophysical properties. Triad 1 emits bright-yellow light in THF solution with the highest quantum yield and the longest decay time among these compounds. It tends to form nanoparticles in aqueous THF solution. The in situ generated nanoparticles might be used to detect silver ions with high sensitivity and high selectivity. By holding the molecules via multiple CH⋯π interactions and electrostatic forces between the tetraethylammonium cation and the cage, a sheet-like arrangement of 1 is formed in the solid state with the smallest crystal density that provides the maximal possibility of mechanofluorochromism from the crystalline to the ground form. Moreover, by doping 1 in PVK, a white OLED is obtained.


 Please wait while we load your content...
Please wait while we load your content...