The effect of manganese oxidation state on antiferromagnetic order in SrMn1−xSbxO3 (0 < x < 0.5) perovskite solid solutions†
Abstract
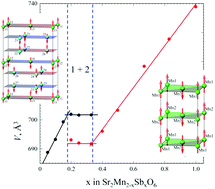
The mixed-valence manganese (Mn3+/Mn4+) solid solution, SrMn1−xSbxO3, was prepared for the first time. Two ranges of solid solutions were found: (1) SrMn1−xSbxO3 (0.025 ≤ x ≤ 0.09) with monoclinically distorted 6H-SrMnO3 polytype (sp. gr. C/2c) and (2) SrMn1−xSbxO3 (0.17 ≤ x ≤ 0.50) with a tetragonal unit cell (sp. gr. I4/mcm). Crystal structure refinement using X-ray and neutron powder diffraction data showed that the structure of the monoclinic solid solution consists of corner-sharing octahedra around sites occupied by manganese and antimony ions and face-sharing octahedra around sites occupied by manganese ions only, while the tetragonal solid solution has a random distribution of B-site cations. The presence of long-range antiferromagnetic order with a Néel temperature of about 148 K for SrMn0.80Sb0.20O3 and about 280 K for SrMn0.925Sb0.075O3 was found from the results of DC and AC susceptibility and neutron diffraction experiments at 5 K and 80 K.


 Please wait while we load your content...
Please wait while we load your content...