An all-small-molecule organic solar cell derived from naphthalimide for solution-processed high-efficiency nonfullerene acceptors†
Abstract
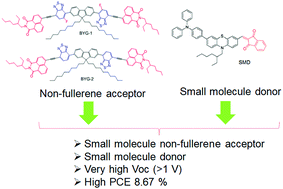
Two small molecules BYG-1 and BYG-2 with fluorene donor and benzothiadiazole acceptor units connected to the terminal naphthamide group via ethyne linker were designed and synthesized. In this work we have discussed the effect of fluorine atoms connected with electron withdrawing benzothiadiazole unit to the fluorene core (BYG-1). In this study, we have fabricated solar cells with small-molecular donor and acceptor materials in the device architecture of bulk-heterojunction, using highly conjugated BYG-1 and BYG-2 as electron acceptors along with an appropriate small molecule donor (SMD). After improving the device architecture of the active layer using a suitable donor-to-acceptor weight ratio with solvent vapour annealing, we achieved power conversion efficiencies of 8.67% and 7.12% for BYG-1 and BYG-2, respectively. The superior photovoltaic performance of the fluorine-substituted BYG-1 can be attributed to its higher crystallinity, more balanced charge transport mobilities and efficient exciton dissociation.


 Please wait while we load your content...
Please wait while we load your content...