Triphenylethylene-based biimidazoles showing preferable detection of explosives and their rhenium complexes undergoing chiral and cis–trans transformations†
Abstract
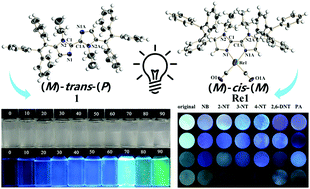
For the first time rhenium-containing biimidazoles have been structurally reported with two triphenylethylene units following chiral and cis–trans transformations simultaneously. Crystal structures show that the biimidazole ligand exhibits an (M)-trans-(P) configuration, whereas the (M)-cis-(M) mode was observed in the rhenium complex, showing cis–trans configurations between biimidazoles and the relative chiral isomers among adjacent triphenylvinylimidazole units. Furthermore, aiming to verify the association rule between spectral behaviours and orientational changes, fluorescence titration experiments were performed for full comparison where the detection performance for explosives in an aqueous phase depends on the alternation of the molecular structure, including the introduction of two alkyl chains and fac-[Re(CO)3Cl]. In addition, computational studies on molecular conformations with different torsion angles for related compounds were carried out.


 Please wait while we load your content...
Please wait while we load your content...