Two isomeric perylenothiophene diimides: physicochemical properties and applications in organic semiconducting devices†
Abstract
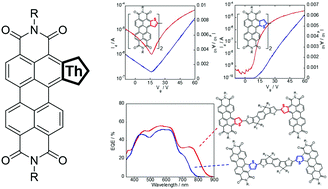
Two thiophene-fused perylene-3,4,9,10-tetracarboxy diimides, peryleno[2,1-b]thiophene diimide (PTIa) and peryleno[1,2-b]thiophene diimide (PTIb), were synthesized as electron-deficient building units for electronic and optoelectronic materials. Both PTIs have an absorption band in the 480–600 nm range, fluorescence emission with high quantum yield (PTIa: ϕflu = 0.82 and PTIb: ϕflu = 0.85) in the 600–700 nm range, and a low-lying LUMO energy level (−3.9 eV). Moreover, the functionalizable α-positions of the fused thiophenes of PTIs allowed us to develop PTI-based π-extended molecules as organic semiconducting materials. Dimerized PTIs (dPTIa and dPTIb) were synthesized for organic field-effect transistors (OFETs), and acceptor–donor–acceptor type triads (IDT-PTIa and IDT-PTIb) were developed as electron-accepting materials for organic photovoltaics (OPVs). The two dPTIs, dPTIa and dPTIb, have different physicochemical properties even though their structural difference is only the direction of the fused thiophenes of the PTI units. The same can be said for the two IDT-PTIs. The PTIa-based materials tend to have higher HOMO energy levels and more bathochromically shifted absorptions than the PTIb-counterparts. The differences in the physicochemical properties affected the properties of the organic devices: dPTIa-based OFETs showed ambipolar behaviors, whereas dPTIb-based ones worked as n-channel unipolar OFETs; IDT-PTIa-based OPVs exhibited NIR photo-response up to 880 nm, whereas IDT-PTIb-based ones showed photocurrent generation only up to 800 nm. These results indicate that PTIa and PTIb are an interesting pair of electron-deficient building units that can tune the electronic structures of resulting organic semiconductors.


 Please wait while we load your content...
Please wait while we load your content...