Zn2+/Cd2+-RNA-mediated intense white-light-emitting colloidal CdSe nanostructures in aqueous medium – enhanced photophysics and porous morphology induced by conformational change in RNA†
Abstract
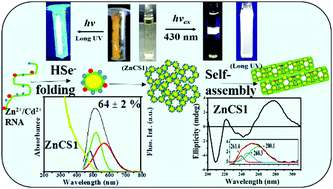
The present work reports the synthesis of Zn2+/Cd2+-bound RNA-mediated CdSe nanostructures, which emit intense white light, in an aqueous medium. A typical stoichiometric ratio of Zn2+ : Cd2+ = 1 : 2 with a relatively low concentration of Zn2+ ions (1.5 × 10−4 mol dm−3) induced the puckering of sugar moieties in RNA bound to the surface of CdSe to bring about an effective conformational change from its B form to its thermodynamically stable A form, as was revealed by IR, NMR and CD spectroscopy. In the early stages, it controlled the nucleation of CdSe QDs without forming an additional ZnSe phase. During self-assembly these QDs were encapsulated by an RNA strand to produce flute-like nanoarchitectures via the formation of porous building blocks. The average size of the core CdSe QDs was reduced from 3.5 nm in a freshly prepared sample to about 1 nm in an aged sample. The addition of Zn2+ ions regularly increased the blue-shifted band edge fluorescence and fluorescence lifetime to induce an intense white-light emission via a yellow emission during self-assembly. In this process, Zn2+/Cd2+ ions bound to folded RNA effectively passivated the surface of CdSe via non-covalent interactions as well as by the entry of Zn2+ ions into the octahedral voids to increase the fluorescence quantum efficiency to 64 ± 2%. The fluorescent nanostructures could be utilized for the selective sensing of Cu2+ ions at concentrations of ≤10 nM in the presence of other interfering bivalent metal ions (Mn2+, Ni2+, Pb2+ and Co2+). I–V plots of a drop-cast thin film of a fresh CdSe sample indicate its symmetric nature, which became asymmetric in a self-assembled sample.


 Please wait while we load your content...
Please wait while we load your content...