Twisted intramolecular charge transfer and aggregation-enhanced emission characteristics based quinoxaline luminogen: photophysical properties and a turn-on fluorescent probe for glutathione†
Abstract
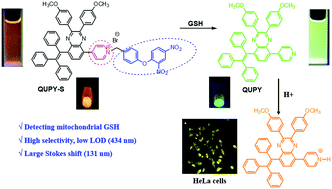
Developing solid and aggregate state emitters with large Stokes shifts has long been a significant challenge in the detection and bioimaging fields. In this study, a novel quinoxaline-based luminogen (QUPY) with twisted intramolecular charge transfer (TICT) and aggregation-enhanced emission (AEE) characteristics has been designed and synthesized. Its pyridine salt derivative (QUPY-S) was also AEE-active and could be utilized as a fluorescent “turn-on” probe for the specific detection of glutathione (GSH). The cleavage of dinitrophenyl ether from QUPY-S by GSH generated AEE-active and less water-soluble QUPY, which exhibited a “turn-on” fluorescence response at around 516 nm. This reaction-based probe showed a large Stokes shift (131 nm), low detection limit (434 nM), fast response time, and low toxicity. QUPY-S was successfully applied for the detection of GSH in bovine serum samples with recoveries ranging from 91.9% to 100.7%. Additionally, QUPY-S could detect GSH in HeLa cells by confocal laser scanning microscopy.


 Please wait while we load your content...
Please wait while we load your content...