Fluorinated hexagonal 4H SrMnO3: a locally disordered manganite
Abstract
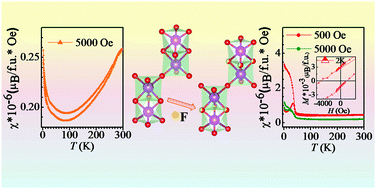
The structural, magnetic and dielectric properties of the hexagonal four-layered form of SrMnO3 along with its fluorinated counterpart have been investigated. Among these, the fluorinated hexagonal four-layered compound is being reported for the first time. Although the bulk crystal structure does not change with fluorination, there are clear spectroscopic evidences of local chemical fluorination having disordered Mn–F rich phases in the fluorinated compound. Fluorination of bulk antiferromagnetic SrMnO3 changes the magnetic properties considerably where a spin- or cluster-glass-like state appears below 42 K with a large exchange bias. In addition to that, the F-doped SrMnO3 indicates development of polarization and the system exhibits ferroelectric nature which has been confirmed through the strain–electric field (S–E) hysteresis loop.


 Please wait while we load your content...
Please wait while we load your content...