PEGylated gold nanoparticles promoted rapid macromolecular chain-end transformation and formation of injectable hydrogels†
Abstract
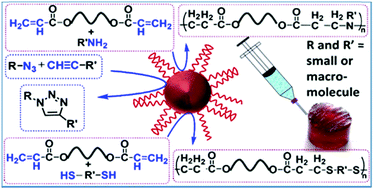
Azide–alkyne click cycloaddition and Michael addition reactions are useful for the synthesis and modification of biologically relevant macromolecules. Acceleration of these reactions at the macromolecular chain-ends and backbone has been achieved by gold [Au-(PEG-SH)n] nanoparticles (NPs) stabilized with multi-thiol-functional poly(ethylene glycol) containing tertiary amine groups in its backbone. The Au NPs successfully activate electron rich alkyne and acrylate functionalities of macromolecules at low substrate concentration leading to the enhancement of the reaction rate. The Au NPs successfully accelerate the gelation rate of reactive prepolymers leading to the rapid formation of injectable hydrogels. The Au nanocomposite hydrogels exhibited higher ultimate modulus and porosity than those of the pristine hydrogels. The grafting density (chains per nm2) of the stabilizer onto the Au NP surface plays a crucial role towards the activity of the NPs. The conversion of the chain-end functionality and gelation rate increase with decreasing grafting density onto the NP surface. A high grafting density lowers the activity of the Au NPs through blocking the active metal surface. The developed Au NPs may be a potential agent for the rapid preparation of biologically relevant macromolecular entities.


 Please wait while we load your content...
Please wait while we load your content...