Sharp pH-responsive mannose prodrug polypeptide nanoparticles encapsulating a photosensitizer for enhanced near infrared imaging-guided photodynamic therapy†
Abstract
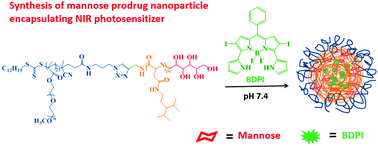
Mannose has been reported as a novel drug to kill cancer cells. The prodrug of mannose will promote its targeted delivery and enrichment at the tumor site and cancer cells. Here, a pH-sensitive polypeptide copolymer with a tertiary amine group has been prepared and a mannose molecule was conjugated to the polymer through the formation of a Schiff base. At the same time, an iodinated boron dipyrromethene (BDPI) photosensitizer with high singlet oxygen generation efficacy and near infrared (NIR) fluorescence was encapsulated by the nanoparticles, which makes it a potential pH-sensitive NIR imaging-guided chemotherapy/PDT agent. In vitro and in vivo studies reveal that in a tumor acidic environment, the protonation of the tertiary amine group destroyed the nanostructure of the nanoparticles, resulting in increased BDPI release. Meanwhile, the bond cleavage of the Schiff base led to the release of conjugated mannose and synergistic inhibition of tumor cell growth with the PDT effect was realized. The combination of these two kinds of tumor suppression effects and photodynamic therapy made this pH-sensitive polypeptide delivery system show great potential for further cancer therapy.


 Please wait while we load your content...
Please wait while we load your content...