Glutamine-β-cyclodextrin for targeted doxorubicin delivery to triple-negative breast cancer tumors via the transporter ASCT2†
Abstract
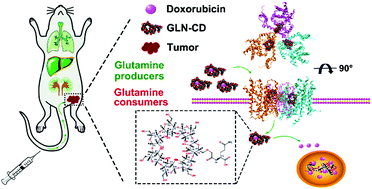
Chemotherapy is the primary therapy for triple-negative breast cancer (TNBC) and the tumor-targeted delivery of chemotherapeutic drugs is necessary to minimize their side effects on normal tissues. TNBC cells display addictions to glutamine in culture, and the levels of the glutamine transporter, alanine–serine–cysteine transporter 2 (ASCT2), are elevated in many types of cancer. However, glutamine- or ASCT2-based carriers have not been used in tumor-targeted drug delivery. In this study, a novel derivative of β-cyclodextrin (β-CD), glutamine-β-cyclodextrin (GLN-CD), was developed by conjugating glutamine with the 6-hydroxy of β-CD, and GLN-CD was then used to prepare doxorubicin (DOX) inclusion complexes (DOX@GLN-CD) for TNBC treatment. GLN-CD and glutamine have similar ASCT2-binding sites, and GLN-CD has the potential to enter cells through ASCT2-dependent facilitated diffusion. An increase in the degree of substitution did not promote binding between GLN-CD and ASCT2. GLN-CD and DOX formed inclusion complexes at a molar ratio of 1 : 1. DOX@GLN-CD specifically accumulated in TNBC cells, including MDA-MB-231 and BT549 cells, where it subsequently induced G2/M blockade and apoptosis, but hardly affected nontumorigenic MCF10A cells. L-γ-Glutamyl-p-nitroanilide (GPNA), which is a specific inhibitor of ASCT2, antagonistically decreased the cellular uptake of DOX@GLN-CD by TNBC cells, which further confirmed the role of ASCT2 in DOX@GLN-CD transport. In vivo, DOX@GLN-CD accumulated specifically in tumors, achieved improved outcomes and minimized the toxic effects on main organs at the same dose as DOX. As a novel derivative of β-CD, GLN-CD is an effective carrier that can specifically deliver DOX to TNBC cells via targeting ASCT2 and minimize its uptake by normal cells.


 Please wait while we load your content...
Please wait while we load your content...