Mannose-coated polydiacetylene (PDA)-based nanomicelles: synthesis, interaction with concanavalin A and application in the water solubilization and delivery of hydrophobic molecules†
Abstract
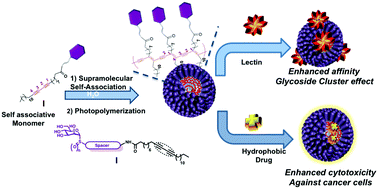
Carbohydrate–lectin interactions are involved in a number of relevant biological events including fertilization, immune response, cell adhesion, tumour cell metastasis, and pathogen infection. Lectins are also tissue specific, making carbohydrates not only promising drug candidates but also excellent low molecular weight ligands for active drug delivery system decorations. In order for these interactions to be effective multivalency is essential, as the interaction of a lectin with its cognate monovalent carbohydrate epitope usually takes place with low affinity. Unlike the covalent approach, supramolecular self-assembly of glyco-monomers mediated by non-covalent forces allows accessing multivalent systems with diverse topology, composition, and assembly dynamics in a single step. In order to fine-tune the size and sugar adaptability of spherical micelles at the nanoscale for an optimal glycoside cluster effect, herein we report the synthesis of mannose-coated static micelles from diacetylene-based mannopyranosyl glycolipids differing in the length of the poly(ethyleneglycol) (PEG) chains and the oxidation state of the anomeric sulfur atom. The reported shot-gun like synthetic approach for the synthesis of dilution-insensitive micelles is based on the ability of diacetylenic-based neoglycolipids to self-assemble into micelles in water and to undergo an easy photopolymerization by a simple irradiation at 254 nm. The affinity of the obtained 6 nanosystems was assessed by enzyme-linked lectin assay (ELLA) using the mannose-specific concanavalin A lectin as a model receptor. Relative binding potency enhancements, compared to methyl α-D-mannopyranoside used as control, from 20-, to 29- to 300-fold on a sugar molar basis were observed for micelles derived from sulfonyl-, sulfinyl- and thioglycoside monomers with a tatraethyleneglycol spacer, respectively, indicative of a significant cluster glycoside effect. Moreover, pMic1 micelles are able to solubilize and slowly liberate lipophilic clinically relevant drugs, and show the enhanced cytotoxic effect of docetaxel toward prostate cancer cells. These findings highlight the potential of mannose-coated photopolymerized micelles pMic1 as an efficient nanovector for active delivery of cytotoxic hydrophobic molecules.


 Please wait while we load your content...
Please wait while we load your content...