Reversible immobilization of a protein to a gold surface through multiple host–guest interactions†
Abstract
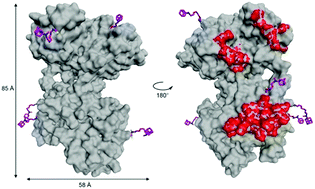
Monolayers were formed by specific interactions between adamantylated proteins (transferrin, lysozyme) and a β-cyclodextrin (β-CD) monolayer on a gold surface. Very high stabilities could be reached by multiple interactions of 3–6 adamantyl moieties linked through triethylene glycol spacers to the protein with β-CD rings attached to the surface. Furthermore, bound proteins could be completely removed from the surface through competitive binding of an excess of free adamantane. Regenerable protein sensor chips can be constructed by using this supramolecular toolbox. Attached proteins are still recognized by specific antibodies, which was attributed to a loose packing of the protein molecules at the β-CD monolayer.


 Please wait while we load your content...
Please wait while we load your content...