Constructing peptide-based artificial hydrolases with customized selectivity†
Abstract
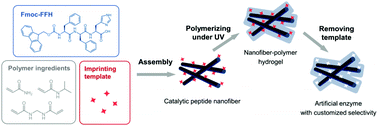
Peptide-based materials are promising building blocks for the fabrication of artificial enzymes due to their proteic nature and tailorable structure. However, it is still a big challenge to create artificial enzymes with designable selectivity. In this study, the molecular imprinting strategy was combined with the self-assembly of Fmoc-FFH to create an artificial hydrolase with specific selectivity. By using p-NPA as the imprinting template, we found that the imprinted polymer that was coated on the surface of the catalytic peptide nanofibers provided a specific binding site to p-NPA, hence enhancing the catalytic efficiency of the artificial hydrolase. Furthermore, we confirmed that the substrate selectivity of this artificial enzyme can be customized by changing the imprinting template. The obtained catalyst is recyclable and exhibits a higher catalytic activity at a wider reaction temperature and pH due to the introduction of the polymer. This study provides a new approach to constructing enzyme mimics with customized substrate selectivity based on the peptide-based material platform.


 Please wait while we load your content...
Please wait while we load your content...