An arsenic trioxide nanoparticle prodrug (ATONP) potentiates a therapeutic effect on an aggressive hepatocellular carcinoma model via enhancement of intratumoral arsenic accumulation and disturbance of the tumor microenvironment†
Abstract
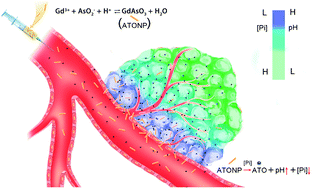
The past decades have seen rapid developments in nanomedicine, but the therapeutic efficacy of nanosized anti-cancer agents remains marginal, although some have reached the clinic “bedside”. Nano-assembly, as a versatile and multi-stage platform, not only contributes to a higher anticancer drug delivery, but also provides synergistic effects (e.g., integrative therapy, theranostics, etc.). We have designed a “Pi-activated” arsenic trioxide (ATO) pro-drug (ATONP) based on colloidal gadolinium arsenite nanoparticles and now present evidence for chemotherapeutic efficacy on a transgenic hepatocellular carcinoma model. The colloidal prodrug was synthesized under mild conditions. After administration of the ATO nanoparticle prodrug, arsenic accumulation within tumors was found to reach as much as 5%, which is ten times higher than following administration of pure ATO. Moreover, the interstitial inorganic phosphate decreased, and the corresponding pH was elevated, as shown in a series of tests in vitro and in vivo. We used a clinically relevant Tet-Off MYC inducible transgenic liver cancer model to evaluate the therapeutic effect. In the first cohort, the tumor volume decreased more than 50% after multiple doses. In the second cohort, ATONP significantly prolonged mouse survival compared with control treatment groups (ATO, sorafenib and saline). Furthermore, the toxicity of the ATONP is reversible. These results suggest that ATONP is a potential drug for the treatment of liver cancer.


 Please wait while we load your content...
Please wait while we load your content...