Dual modulation of crystallinity and macro-/microstructures of 3D printed porous titanium implants to enhance stability and osseointegration
Abstract
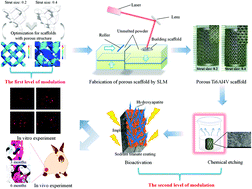
The macro architecture and micro surface topological morphology of implants play essential roles in bone tissue regeneration. 3D printing technology provides enormous advantages for the rapid fabrication of personalized bone tissue repair implants. This study presents a demonstration of dual-modulation (DM) 3D printed porous titanium implants to enhance stability and osseointegration. Titanium implants with the first level of modulation of macro porous architecture and mechanical properties are obtained using macro architecture design and 3D printing fabrication. The first level of modulation achieved scaffolds with a wide range of compressive strengths (36.76–139.97 MPa) when varying the scaffold macro architectures. In the second level of modulation of surface topological morphology, alkali treatment, heat treatments and electrochemical deposition of hydroxyapatite coating were conducted for further regulating the biological function of implants. DM 3D printed scaffolds significantly promoted bone marrow mesenchyme stem cell adhesion and proliferation, indicating good cytocompatibility. In addition, in vivo osseointegration experiments indicated that the DM scaffolds formed better tissue–materials interfaces. New bone formation rates in DM scaffolds are higher than those in conventional 3D printed scaffolds after 6 months of implantation (58.1% versus 36.1%). These results demonstrate that DM scaffolds could enhance early stability and osseointegration. This study may provide new insights into the design, fabrication and post-processing of 3D printed porous titanium implants for various applications in personalized bone tissue regeneration.


 Please wait while we load your content...
Please wait while we load your content...