A self-assembling collagen mimetic peptide system to simultaneously characterize the effects of osteogenesis imperfecta mutations on conformation, assembly and activity†
Abstract
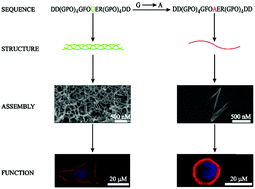
Osteogenesis imperfecta (OI) is a hereditary connective tissue disorder occurring mainly due to missense mutations in collagen, which has complicated effects on stability and conformation of monomer collagen as well as the morphology and bioactivity of aggregated collagen. We have created a self-assembling collagen mimetic peptide system that for the first time facilitates simultaneous characterization of the effects of osteogenesis imperfecta mutations on stability, conformation, assembly and activity. The constructed collagen mimetic peptide with an integrin binding motif in the center and aspartic amino acids at two terminals maintains a classic triple helix structure, and self-assembles to form exquisite nanofibers characteristic of native collagen under the trigger of lanthanide ions. The biocompatible peptide–lanthanide assemblies exhibit good cell adhesion and spreading features, suggesting that this novel self-assembling peptide system can simultaneously well mimic the triple helix structure, and the assembly and function of native collagen. The introduction of a Gly–Ala mutation in the peptide has been found to result in a disrupted triple helix structure, abnormal assembly and loss of activity. These results have demonstrated that the self-assembling collagen peptide system provides a robust platform to evaluate OI mutations at multiple levels varying from solution structure to assembly and function. The system may be expanded to investigate a variety of collagen interactions, and it would greatly contribute to our insights into collagen-related diseases and therapies.


 Please wait while we load your content...
Please wait while we load your content...