Single-atom catalysts templated by metal–organic frameworks for electrochemical nitrogen reduction†
Abstract
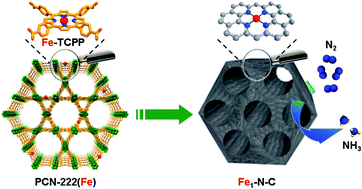
The electrocatalytic nitrogen reduction reaction (NRR) has much prospect for substituting the energy-consuming Haber–Bosch process. Nevertheless, its sluggish reaction kinetics and the competing hydrogen evolution reaction always result in limited ammonia yield and low faradaic efficiency (FE). In this work, an Fe-decorated porphyrinic metal–organic framework (MOF) is employed as a precursor to construct single-atom Fe implanted nitrogen-doped carbon catalysts (Fe1-N-C) through a mixed ligand strategy. Benefiting from the highly dispersed single-atom Fe sites, hierarchically porous structure and good conductivity, Fe1-N-C shows a FE of 4.51% and an ammonia yield rate of 1.56 × 10−11 mol cm−2 s−1 at −0.05 V versus the reversible hydrogen electrode, superior to those of Co1-N-C and Ni1-N-C. Theoretical calculations reveal that Fe1-N-C shows the lowest energy barrier of the rate-determining step during the NRR process, consistent with its highest activity obtained in experiments. This work reveals the unique potential of single-atom catalysts for the electrochemical NRR and provides in-depth insights into the catalytic mechanism of the NRR.


 Please wait while we load your content...
Please wait while we load your content...