Chemically coupled NiCoS/C nanocages as efficient electrocatalysts for nitrogen reduction reactions†
Abstract
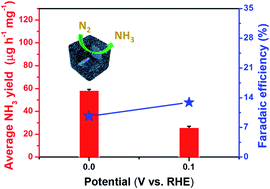
Ammonia (NH3) as a significant industrial material as well as a clean energy carrier has become the center of attention. Using electrocatalysis to convert N2 to NH3 at ambient conditions is an attractive method among other sustainable methods. However, achieving high yields of NH3 and high Faraday efficiency (FE) at a low overpotential is still a big challenge in electrocatalytic N2 reduction reactions (NRRs). In this study, strongly chemically coupled NiCoS/C nanocages were first used for the conversion of N2 to NH3 in the electrocatalytic NRR. Benefiting from the unique structure and strong chemical coupling between C and NiCoS, the NiCoS/C nanocages exhibit superior NRR performance and selectivity in 0.1 M Li2SO4. It can achieve a high ammonia production rate of 26.0 μg h−1 mg−1 (2.60 μg h−1 cm−2) and a high FE of 12.9% at 0 V (vs. RHE). Notably, with the increasing potential, the ammonia production rate could reach the highest value of about 58.5 μg h−1 mg−1 (5.85 μg h−1 cm−2). The density functional theory reveals that the strong chemical coupling effect between NiCoS and C is of great importance in lowering the NRR overpotential and enhancing the electrochemical NRR activity and selectivity. This work provides a new avenue for designing the strongly chemically coupled NRR electrocatalysts to realize high NRR performance at a low overpotential.


 Please wait while we load your content...
Please wait while we load your content...