Revealing cooperative Li-ion migration in Li1+xAlxTi2−x(PO4)3 solid state electrolytes with high Al doping†
Abstract
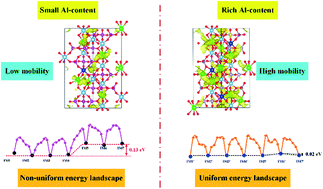
Li1+xAlxTi2−x(PO4)3 (LATP) is attracting attention as a promising inorganic solid electrolyte (ISE) with potential use in all-solid-state lithium-ion batteries. The objective of this paper is to examine and understand the effect of the Al-dopant concentration on the Li-ion diffusion of LATP using density functional theory and the molecular dynamics method. By comparing Li1.16Al0.16Ti1.84(PO4)3 (LATP-0.16) and Li1.33Al0.33Ti1.67(PO4)3 (LATP-0.33) with Li1.5Al0.5Ti1.5(PO4)3 (LATP-0.50), LATP-0.50 is expected to have higher ionic conductivity. The trapping effect of Al-dopants on Li-ions is greatly reduced in LATP-0.50 due to the delocalization of polarization interactions and the depopulation of oxygen atoms, which results in a smooth energy landscape and destabilization of Li-ions. The energy difference of adjacent Li-ions and binding interaction of Li–Li due to a specific local configuration of two Li-ions alternately enable cooperative migration of Li-ions. This understanding of high Li-ion diffusion is important in interpreting the experimental results aiming to assess the effects of Al-dopants on Li-ion conductivity and can be used by researchers to engineer these materials for batteries.


 Please wait while we load your content...
Please wait while we load your content...