A self-healing interface on lithium metal with lithium difluoro (bisoxalato) phosphate for enhanced lithium electrochemistry†
Abstract
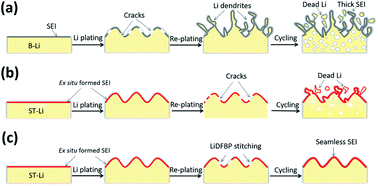
The practical applications of lithium (Li) metal anodes are suppressed by one of the most serious obstacles to energy storage: the high interfacial instability during deposition and dissolution. A novel strategy that simultaneously employs pre-treatment and self-healing of the interface of the lithium metal anode with highly reduced lithium difluoro (bisoxalato) phosphate (LiDFBP) is proposed. With surface treatment in a LiDFBP-containing solution, an ex situ passivation layer is generated on the lithium metal surface. Additive LiDFBP within the electrolyte formulation immediately and continuously reacts to repair cracks in the interfacial layer during dynamic lithium plating/stripping. This thorough interfacial layer is primarily rich in lithium oxalate (Li2C2O4) from the decomposition reactions of LiDFBP, which generate a product that stabilizes lithium metal. This property is responsible for the dendrite-free morphology and enhanced cyclability of cells made with this system. The lithium symmetric cell shows 2 times improvement in cycle lifetime compared to the non-treated/non-additive systems. This improved performance is also observed in asymmetric Li‖LiFePO4 cells with the SOC increasing from 50% to 85%. The strategy described herein addresses these dilemmas of lithium metal electrodes, and presents a scalable way to further promote high-energy-density Li-metal batteries.


 Please wait while we load your content...
Please wait while we load your content...