An in situ electrochemical oxidation strategy for formation of nanogrid-shaped V3O7·H2O with enhanced zinc storage properties†
Abstract
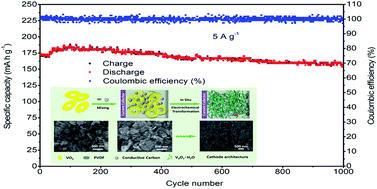
Rational design and synthesis of cathode materials with a well-defined nanostructure and superior performances have always been of paramount importance for rechargeable zinc ion batteries. Here, based on the rearrangement of the morphology and crystal phase structure, uniform and ultrafine V3O7·H2O nanogrids are obtained via in situ conversion of an initial nsutite-type VO2 precursor. Furthermore, benefiting from the structural advantages of the large electrolyte-accessible surface and abundance of mass diffusion pathways, the V3O7·H2O nanogrid acts as a cathode material that delivers a high reversible specific capacity of up to 481.3 mA h g−1 at a current density of 0.1 A g−1, as well as a large specific capacity of 171.6 mA h g−1 with retention of 85.4% after 1000 cycles (high current density of 5 A g−1) for rechargeable zinc ion batteries. These results demonstrate that in situ conversion of composition and morphology simultaneously can be used as a new pathway to prepare high-efficiency electrode materials.


 Please wait while we load your content...
Please wait while we load your content...