Sulfuryl chloride as a functional additive towards dendrite-free and long-life Li metal anodes†
Abstract
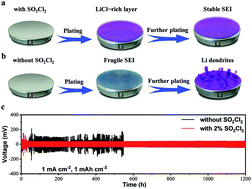
With ultrahigh theoretical specific capacity and the lowest redox potential, lithium (Li) metal is regarded as the most promising anode material for the electrochemical energy storage field. However, the formation of undesired Li dendrites severely hinders its practical application in Li metal batteries (LMBs), during repeated Li plating/stripping processes. Herein, we report that an optimal amount (2%) of sulfuryl chloride (SO2Cl2) could highly improve the electrochemical performance of the Li metal anode in an ester-based electrolyte by in situ forming a stable inorganic protective layer on its surface to strengthen the native solid electrolyte interphase (SEI). The LiCl-rich SEI layer is beneficial for suppressing the growth of Li dendrites and accelerating the migration of Li ions. As a result, a greatly enhanced coulombic efficiency (CE) of 95% after 150 cycles in a Li‖Cu cell and a long-term cycling life of over 1200 h with a low and stable overpotential of ∼40 mV in a Li‖Li symmetric cell have been achieved. Therefore, this work provides a new and promising strategy for speeding up the practical application of Li anodes in LMBs.


 Please wait while we load your content...
Please wait while we load your content...