NASICON vs. Na metal: a new counter electrode to evaluate electrodes for Na secondary batteries†
Abstract
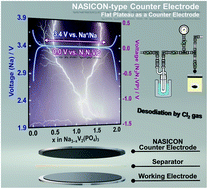
Na metal has been used as a counter electrode in a half-cell configuration to test positive and negative electrode materials for Na secondary batteries. However, there are significant obstacles including high reactivity, which generates a resistive passivation layer along with electrolyte decomposition, dendrite formation that results in poor cyclability, dead Na metal accumulation, which impedes Na+ transport, and a low melting point, which limits its use to below 98 °C. Herein, an alternative counter electrode is devised using NASICON-type Na3V2(PO4)3 to measure the accurate electrochemical behaviour of working electrode materials and to use for measurements above the melting point of Na metal. The novel counter electrode is prepared by mixing Na3V2(PO4)3 and NaV2(PO4)3, and the latter is prepared by the desodiation of Na3V2(PO4)3 using Cl2 gas. The resulting Na3V2(PO4)3–NaV2(PO4)3 electrode exhibits a flat plateau at 3.4 V vs. Na+/Na and lower polarization than the Na metal. The electrochemical behaviours of the Na2FeP2O7, Na3V2(PO4)3, and NaCrO2 electrodes tested with the new counter electrode match the known curves recorded with the Na metal at low current densities and show better cyclability and rate performance. Moreover, the electrochemical properties of these electrode materials are verified at a temperature above the melting point of Na metal for the first time.


 Please wait while we load your content...
Please wait while we load your content...