In situ grown cobalt phosphide (CoP) on perovskite nanofibers as an optimized trifunctional electrocatalyst for Zn–air batteries and overall water splitting†
Abstract
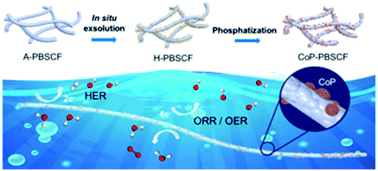
Developing highly efficient and stable multi-functional electrocatalysts is of prime importance for renewable energy technologies. Herein, a trifunctional electrocatalyst featuring CoP socketed on an oxygen deficient layered perovskite is synthesized through an in situ exsolution and an intriguing “post-growth” approach. As revealed by experimental characterization and density functional theory (DFT) simulation, the simultaneous introduction of CoP and oxygen vacancies modifies the electronic configuration of the catalysts and the reactants adsorbed on their surface. The intimate interpenetration of these two components yields synergistically active sites, which results in excellent performances towards the oxygen reduction/evolution reaction (ORR/OER) and hydrogen evolution reaction (HER). The CoP-PrBa0.5Sr0.5Co1.5Fe0.5O5+δ (CoP-PBSCF) electrode demonstrates a high power density (138.0 mW cm−2), a competitive charge/discharge polarization, and a superior stability to commercial Pt/Ru/C in Zn–air batteries. Moreover, it shows a low overpotential (0.440 V) in overall water splitting, surpassing most reported electrocatalysts. The proposed protocol offers an attractive strategy for designing perovskite-based electrocatalysts for versatile devices.


 Please wait while we load your content...
Please wait while we load your content...