Understanding the effects of chemical reactions at the cathode–electrolyte interface in sulfide based all-solid-state batteries†
Abstract
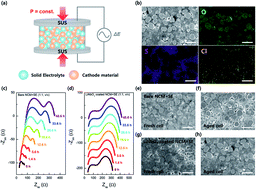
Driven by a paradigm shift from conventional liquid-based systems to all-solid-state batteries (ASSBs), the chemo-mechanical behavior of the solid–solid interface is of growing importance for understanding the intricate interfacial phenomena of ASSBs. During electrochemical cycling, various degradation factors at the interfaces of the cells are complexly intertwined, such as electrochemically driven volume changes of active materials and the formation of a chemically vulnerable highly charged cathode. Moreover, chemical reactions that occur immediately after cell assembly at the interface between the solid materials involving the diffusion of chemical species at the junction are also correlated to the cell performance. However, the individual effects on the electrochemistry of the cell are vaguely understood. In this study, we investigate the independent effect of chemical reactions between a cathode and a sulfide-based electrolyte from complex degradation in ASSBs. We reveal that chemical degradation products are different from those commonly observed after electrochemical cycling and they reduce ionic conduction at grain boundaries. We also find physical contact loss between the cathode and electrolyte, implying that chemically driven mechanical degradations occurred simultaneously. Our research provides an insight into the complex behaviors at the solid–solid interface in sulfide-based ASSBs.


 Please wait while we load your content...
Please wait while we load your content...