Superfast high-energy storage hybrid device composed of MXene and Chevrel-phase electrodes operated in saturated LiCl electrolyte solution†
Abstract
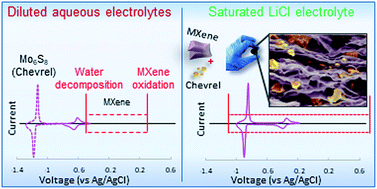
Development of high power devices with improved energy density is a highly desired target for advanced energy storage applications. Herein we propose a new strategy of triply-hybridized supercapacitive energy storage device composed of hybrid battery–supercapacitor negative electrode [Mo6S8 (Chevrel-phase)/Ti3C2 (MXene)] coupled with positive nanoporous carbon electrode, integrated with novel yet unexplored saturated (14 M) aqueous solution of LiCl. The electrochemical stability window of this electrolyte solution (2.70 V) significantly exceeds the cell voltage (2.05 V) relevant for the asymmetrical (hybrid anode vs. carbon cathode) cells. The aqueous 14 M LiCl solution has far superior characteristics to that of the previously studies 21 m LiTFSI aqueous solution. The paper is also focused on a deep electroanalytical analysis of a peculiar redox/capacitive heterogeneity of hybrid electrodes. It establishes a variety of additivity rules for both differential and integral equilibrium and kinetic characteristics of the charging processes in hybrid electrodes, solving the puzzle of potential distribution of specific electrochemical energy stored the hybrid electrodes. A careful 3-level hybridization design of asymmetric supercapacitive storage devices enabled the integration of battery and supercapacitor materials to get free-standing binderless electrodes suitable for high power/high energy density systems. Studying thoroughly the properties of highly concentrated solutions such as aqueous 14 M LiCl, which are very suitable for different types of supercapacitive devices, combined with profound analyses of the properties of hybrid electrodes, will pave the way for a rational design of very effective devices for energy storage and conversion.


 Please wait while we load your content...
Please wait while we load your content...