Accelerated proton transmission in metal–organic frameworks for the efficient reduction of CO2 in aqueous solutions†
Abstract
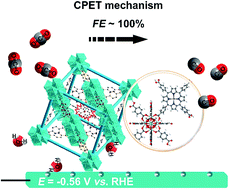
Metal–organic frameworks (MOFs) have exhibited huge potential in the field of CO2 electroreduction but highly stable and active ones are still scarce. This study reports a (5,10,15,20-tetrakis(4-carboxyphenyl)porphyrinato)-Fe(III) chloride (FeTCPPCl) co-building UiO-66 electrocatalyst for CO2 reduction in an aqueous solution. In situ X-ray absorption spectroscopy (XAS) measurements characterized its robust structure under catalysis. The unique framework of UiO-66 provides the available proton facilitator for improving CO2 reduction activity on iron porphyrin, and a concerted proton–electron transfer (CPET) mechanism might be considered for the catalytic pathway, achieving the highest faradaic efficiency of nearly 100% at an overpotential of 450 mV for turning CO2 to CO in the reported MOFs.


 Please wait while we load your content...
Please wait while we load your content...