Sr2Fe1.4Mn0.1Mo0.5O6−δ perovskite cathode for highly efficient CO2 electrolysis†
Abstract
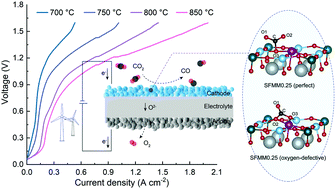
High-temperature solid oxide cells afford chemical storage of renewable electricity. In particular, the electrochemical conversion of the greenhouse gas CO2 is attracting increasing interest to facilitate a sustainable energy technology. In this work, the effectiveness of perovskite-structured Sr2Fe1.4Mn0.1Mo0.5O6−δ (SFMM0.1) for use as cathode material for CO2 electrolysis has been investigated. Both parent Sr2Fe1.5Mo0.5O6−δ (SFM) and SFMM0.1 are found to be redox stable in air and 5% H2/Ar at 850 °C. Electrical conductivity relaxation experiments and first-principle calculations reveal that oxygen transport, CO2 adsorption and reduction kinetics are enhanced upon doping of SFM with Mn. The faster CO2 reduction kinetics observed for SFMM0.1 relative to SFM is reflected in a lower polarization resistance when both materials are used as single-phase electrodes in symmetrical cells. The polarization resistance in 50% CO/CO2 at 800 °C decreases from 1.15 Ω cm2 for SFM to 0.60 Ω cm2 for SFMM0.1. Under similar conditions, the polarization resistance decreases further to 0.50 Ω cm2 for a symmetrical cell with dual-phase SFMM0.1-SDC (samaria-doped ceria) electrodes. Unprecedented performance is demonstrated when SFMM0.1-SDC is integrated as the cathode in a solid oxide cell for electrolysis of pure CO2, achieving a current density of 1.35 A cm2 at 800 °C at 1.5 V.


 Please wait while we load your content...
Please wait while we load your content...