A carbon nanotube-confined iron modified cathode with prominent stability and activity for heterogeneous electro-Fenton reactions†
Abstract
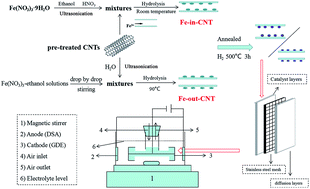
Transition metal modified carbon materials as multifunctional cathodes for electro-Fenton (EF) reactions are supposed to be promising for generating H2O2in situ and catalyzing it to form hydroxyl radicals to degrade organic pollutants, but this process still faces the challenges of reduced heterogeneous catalyst activities and poor stabilities due to the continuous leaching of active metals. Herein, a heterogeneous cathode, in which iron is confined in the interior of carbon nanotube cavities, (Fe0-in-CNTs) with extremely low iron leaching levels was prepared, producing a much higher H2O2 yield and phenol removal rate (9.68 times faster) when compared with iron being confined externally on the walls of CNTs (Fe0-out-CNTs). It was found that the iron valence on the CNTs played an important role in determining the heterogeneous Fenton activity, suggesting that Fe0 was beneficial for H2O2 selectivity through a 2e− process (2.43 times higher) and the phenol removal rate (21.44 times faster) compared to iron oxide. It was confirmed that the CNT cavities could provide an isolated space for Fe0, and the iron leaching mass was only 3.21 × 10−3 mg cm−2, even at pH 3. Consequently, different mechanisms for phenol degradation via heterogeneous EF reactions on Fe0-in-CNT and Fe0-out-CNT cathodes were disclosed. This supported the idea that a heterogeneous Fenton-like reaction on the CNT surface, rather than a solution homogeneous Fenton reaction, played a decisive role in pollutant degradation. Furthermore, the cathode reusability was proved to be dependent on the Fe0 content and efficient conversion between FeIII and FeII. This work verified the importance of confinement catalysis in selectively controlling the positions and valences of iron on CNTs, which could effectively increase the heterogeneous EF activity and decrease the amount of leached iron to improve cathode stability. Thereby this could lead to a significant breakthrough in heterogeneous EF studies and shed light on confinement catalysis methods for organic pollutant degradation.


 Please wait while we load your content...
Please wait while we load your content...