Zinc-telluride nanospheres as an efficient water oxidation electrocatalyst displaying a low overpotential for oxygen evolution†
Abstract
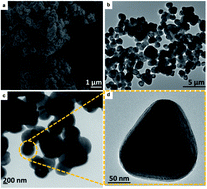
Electrochemical water splitting is economically unviable due to the sluggish kinetics of the anodically uphill oxygen evolution reaction (OER). This is directly linked to the design and facile fabrication of low overpotential water oxidation electrocatalytic materials, engaging applied methods and earth-abundant precursors. Here, we demonstrate facilely synthesized ZnTe nanospheres (ZnTe-NS) as an efficient and durable OER electrocatalyst, executing water oxidation with a much lower energy cost relative to other previously reported non-noble metal-based catalytic systems. The catalytic system shows excellent performance, initiating the oxygen evolution reaction at just 1.41 V vs. RHE (η = 180 mV) and reaching current densities of 10 mA cm−2 and 100 mA cm−2 at 1.44 V vs. RHE (η = 210 mV) and 1.63 V vs. RHE (η = 400 mV), respectively; this performance is superior to state-of-the-art RuO2 and IrO2 catalysts reported previously. Furthermore, a modest Tafel slope of 62 mV dec−1, indicative of a one electron and one proton transfer mechanism, and a large TOF of 0.25 s−1 at just η = 210 mV are achieved. The catalyst advantageously exhibits a stable current density of >50 mA cm−2 for more than 24 hours during water electrolysis experiments for extended time periods. The hydrothermally developed ZnTe-NS are 150–200 nm in size and display hollow structures, as evaluated via high-resolution transmission electron microscopy. XRD and XPS techniques are employed to confirm the formation of ZnTe-NS. DFT calculations show that hydroxyl attachment energy is very favorable on the highly porous and crystalline ZnTe-NS, further confirming their remarkable properties for enhanced electrocatalytic OER activity. This study demonstrates valuable insights into developing the first example of a highly durable Zn chalcogenide, more specifically ZnTe-NS, as an efficient and inexpensive electrocatalyst material for water oxidation. Through an easily accessible synthesis method, ZnTe-NS maintain their integrity, morphology and chemical structure, even after many hours of continuous water electrolysis.


 Please wait while we load your content...
Please wait while we load your content...