Ce-doped CoS2 pyrite with weakened O2 adsorption suppresses catalyst leaching and stabilizes electrocatalytic H2 evolution†
Abstract
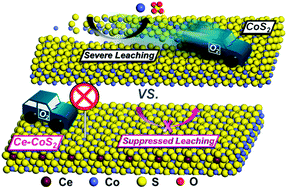
Leaching of pyrite-type catalysts induced by surface oxidation and galvanic corrosion is a sign of poor electrocatalytic durability and limits their potential for the electrocatalytic hydrogen evolution reaction (HER). Weakened O2 adsorption on pyrites can suppress both oxidation and corrosion, preventing the loss of electroactive components and maintaining their activity. Herein, we report our strategy theoretically and experimentally to establish an engineered CoS2 surface by Ce-doping which exhibits improved electrochemical stability against galvanic corrosion and enhanced chemical stability against ambient surface oxidation for an efficient and robust HER in alkali over 250 h. The Ce-doped CoS2 electrode delivered an activity–durability factor of ∼31 times higher than that of the CoS2 electrode, indicating the highly stabilized HER activity. Characterization tests on the spent Ce-doped CoS2 showed negligible corrosion current and leached Co ions in the electrolyte, further demonstrating that the Ce-doping protected CoS2 from oxidation- and corrosion-induced leaching.


 Please wait while we load your content...
Please wait while we load your content...