C–H activation derived CPPs for photocatalytic hydrogen production excellently accelerated by a DMF cosolvent†
Abstract
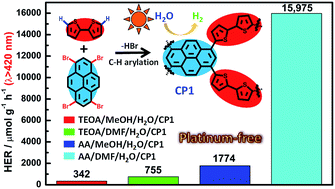
A series of four conjugated porous polymer CPPs (CPs1–4) with various geometries have all been readily synthesized via direct C–H arylation between bithiophene (BT) and 1,3,6,8-tetrabromopyrene (TBPy), 2,2′,7,7′-tetrabromo-9,9′-spirofluorene (TBSF), 1,3,5-tribromobenzene (TBB) and 1,4-dibromobenzene (DB) respectively. The photocatalytic hydrogen evolution from water splitting by these CPPs under visible light irradiation is studied in detail. An amazing hydrogen evolution rate (HER) of 95.85 μmol h−1 was successfully achieved by the four-directional Py-BT-based CPP (CP1, 6 mg), using ascorbic acid (AA) and N,N-dimethylformamide (DMF)/water as a sacrificial agent and co-solvents respectively. Such a HER value is 8-fold higher than that of applying commonly used MeOH/water as a co-solvent in the same photocatalytic system. Significantly, when a 0.5 wt% platinum (Pt) co-catalyst was added into the above photocatalytic system, an unprecedented HER of 184.86 μmol h−1 (6 mg) was further realized. The combination of atom-economically synthesized CPPs and accelerating the HER by means of simply tuning the composition of co-solvents provided herein represents a promising step toward accessing green energy by green chemistry.


 Please wait while we load your content...
Please wait while we load your content...