Comparisons of WO3 reduction to HxWO3 under thermochemical and electrochemical control†
Abstract
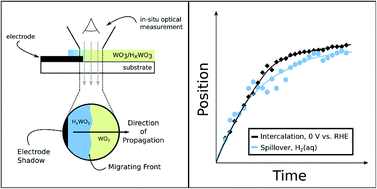
The use of renewable electricity as an energy source for the chemical industry is attractive from the standpoint of improved environmental sustainability. The development of such electrification strategies would greatly benefit from improved understanding of the relationships between thermochemical and electrochemical redox processes. We have used time-resolved optical microscopy to study HxWO3 bronze formation from WO3via thermochemical H-spillover and electrochemical H-intercalation reactions. The rates of hydrogen uptake in these films were found to be similar in each case, and too fast to be limited by proton diffusion in the solid state. These results point to a single predominant reaction mechanism that is primarily gated by interfacial electron-transfer rather than proton or hydrogen-atom diffusion.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...