Selective electrochemical reduction of carbon dioxide to formic acid using indium–zinc bimetallic nanocrystals†
Abstract
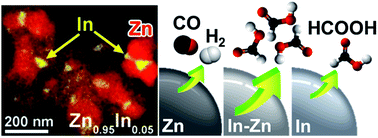
For the electrochemical reduction of CO2 (CRR) with high selectivity for HCOOH, In–Zn bimetallic nanocrystals (NCs) were synthesized as catalysts by in situ reduction of In2O3–ZnO NCs with various compositions. All In-containing bimetallic catalysts exhibited excellent selectivity to produce HCOOH, while Zn NCs favor CO production. A composition with In : Zn = 0.05 has higher catalytic activity than In NCs, with a faradaic efficiency of 95% and a HCOOH production rate of 0.40 mmol h−1 cm−2 at −1.2 V vs. RHE. The enhanced catalytic performance is in part ascribed to the fewer surface oxide layers, which increase the conductivity and facilitate the charge transfer. Density functional theory calculations revealed that the In–Zn interfacial sites make the HCOOH pathway significantly energy-favorable, which supports the higher production rate of Zn0.95In0.05 than that of In.


 Please wait while we load your content...
Please wait while we load your content...