Boron and nitrogen co-doped porous carbon nanofibers as metal-free electrocatalysts for highly efficient ammonia electrosynthesis†
Abstract
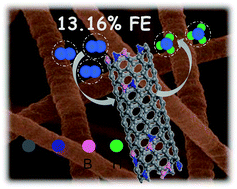
NH3 synthesis via the electrocatalytic nitrogen reduction reaction (NRR) under ambient conditions has been regarded as a sustainable strategy for fertilizer production and hydrogen fuel storage and mitigating greenhouse gas emissions. The faradaic efficiency (FE) and reaction rate of the NRR, however, are still low for current electrocatalysts. Herein, we develop a metal-free B, N co-doped porous carbon nanofiber (B/N–CNF) that exhibits superior electrocatalytic performance for the NRR in an alkaline electrolyte with the highest FE of 13.2% at −0.5 V and a NH3 yield rate of 32.5 μg h−1 mgcat.−1 at −0.7 V. Such a high NRR activity of the B/N–CNF is superior to that of N- or B-doped carbon materials in the literature. The experimental results reveal that the B–N bonds play a critical role in the NRR process, in which N atoms promote the electron conductivity and B atoms efficiently enhance the N2 adsorption and charge transfer.


 Please wait while we load your content...
Please wait while we load your content...