Diversities of stoichiometry and electrical conductivity in sodium sulfides†
Abstract
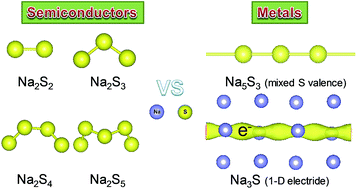
Aiming at developing and understanding the active materials in the renewable energy-storage sodium–sulfur (Na–S) system, we systemically explored the phase diversity, electronic properties, and chemical bonding of the Na–S system at ambient and high pressure up to 50 GPa, using a combination of first-principles calculations with extensive structural searches. We identified four new stable phases; Na3S, Na5S3, Na2S2 and Na2S3. The previously unidentified Na2S3 with S32− polyanions shows Pnma symmetry with quite a low formation enthalpy relative to Na2S2 and Na2S4. The simulated voltage through Na2S4 to generate Na2S3 in a battery is predicted to be 1.65 V, consistent with previous measurements. The predicted tetragonal Na5S3 with infinite S chains has mixed valence states of sulfur atoms, which was first observed in alkali metal sulfides. Na3S exhibits a potential one-dimensional (1-D) electride with the chemical formula of [Na3S]+·e−, where the sulfur atoms possess the highest coordination number (Na12S). Both Na3S and Na5S3 exhibit intrinsic metallic behaviors, clearly differing from other semiconducting phases. A detailed analysis of the electronic structure reveals the distinct electrical pathways of a 1-D electron gas in the channel voids in Na3S and infinite sulfur chains with metallic S–S bonding in Na5S3. Our results may help to discover new candidates in Na–S systems and elucidate the potential electrochemical mechanism in the Na–S battery.

 Please wait while we load your content...
Please wait while we load your content...