Tailoring the ORR and HER electrocatalytic performances of gold nanoparticles through metal–ligand interfaces†
Abstract
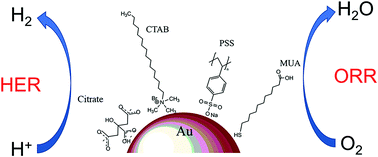
The oxygen reduction (ORR) and hydrogen evolution (HER) reactions are the most important cathodic processes involved in fuel cell and water splitting technologies, respectively. The development of bifunctional electrocatalyst materials plays a key role in the rapid advancement of these hydrogen-based renewable energy strategies. This work proposes citrate-stabilized gold nanoparticles (AuNPs) as a bifunctional electrocatalyst for ORR and HER. The capping ligand has a great influence on their resulting electrocatalytic performance. A simple ligand exchange method based on concentration gradients has been optimized. The surface structure of the different ligand-stabilized AuNPs was inferred by lead underpotential deposition (Pb-UPD). Static and dynamic electrochemical studies for both ORR and HER have been performed using different ligand-stabilized AuNPs as electrocatalysts, demonstrating that the citrate ligand confers the best performance. This work suggests that non-doped chemically synthesized AuNPs may be suitable as a bifunctional electrocatalyst in fuel cells and hydrogen production.


 Please wait while we load your content...
Please wait while we load your content...