Lithiophilic Ag/Li composite anodes via a spontaneous reaction for Li nucleation with a reduced barrier†
Abstract
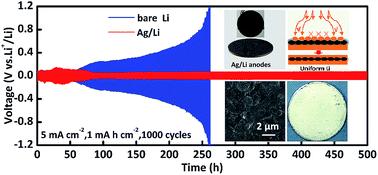
Infinite volume expansion and high reactivity severely hinder the commercial applications of lithium metal batteries. Moreover, the short circuit caused by Li dendrites is fatal for the long-term performance of lithium metal batteries and may cause safety problems. Herein, lithiophilic silver/lithium (Ag/Li) composite anodes are synthesized via a spontaneous displacement reaction between silver nitrate and Li foil at room temperature, which can settle the above challenges by regulating Li nucleation and homogenizing Li-ion flux. Benefiting from the lithiophilic design, Li ion realizes zero nucleation overpotential on the as-obtained Ag/Li anodes with abundant spherical silver nanoparticles. Additionally, the by-product, lithium nitrate, acts as an electrolyte additive, which further ensures uniform Li deposition. Consequently, Ag/Li symmetric cells demonstrate ultralong lifespan with repeated plating/stripping for 1000 cycles at the current densities of 1 mA cm−2 and 5 mA cm−2, with negligible voltage fluctuations. Furthermore, the full-cell devices, paired with LiFePO4, also exhibit excellent electrochemical performance. The facile and effective strategy provides a promising prospect for commercial applications of lithium metal batteries.


 Please wait while we load your content...
Please wait while we load your content...