An on-demand solar hydrogen-evolution system for unassisted high-efficiency pure-water splitting†
Abstract
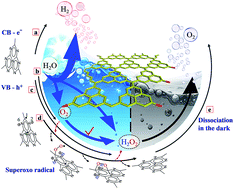
Solar water splitting of pure water offers an attractive means for sustainable and carbon-free H2 production. However, current photocatalytic H2 production systems still suffer from two basic issues: the kinetic bottleneck for O2 release and the easy toxicity of the photocatalysts. Here, we developed a consolidated photocatalyst, namely g-C3N3.5(O0.5H0.5), that can boost sustainable and superior H2 evolution without using any sacrificial reagent. By conceptual design analysis as the guideline for three synthetic steps, the surface hydroxylation structure of g-C3N3.5(O0.5H0.5) concurrently increases the toxicity resistance and maximizes the charge separation for H2 evolution. The obtained surface-hydroxylated g-C3N3.5(O0.5H0.5) photocatalyst exhibited unassisted high-efficiency pure-water-splitting activity, with a benchmark H2-evolution rate up to 947.7 μmol h−1 g−1 under visible-light irradiation. Notably, the quantum efficiency of the g-C3N3.5(O0.5H0.5) suspension reached ∼10.6 and ∼2.5% at 420 and 520 nm, respectively, 10–20 times that of pristine g-C3N4. For the first time, we observed a key phenomenon that H2O molecules can rapidly capture the photoexcited holes to produce H2O2, which can greatly promote the efficient charge separation for high H2 evolution.


 Please wait while we load your content...
Please wait while we load your content...