Origin of the heat-induced improvement of catalytic activity and stability of MnOx electrocatalysts for water oxidation†
Abstract
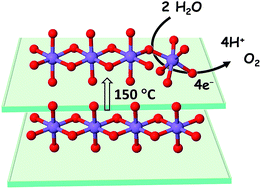
Catalysis of the oxygen evolution reaction (OER) by earth-abundant materials in the near-neutral pH regime is of great interest as it is the key reaction for non-fossil fuel production. To address the pertinent stability problems and insufficiently understood structure–activity relations, we investigate the influence of moderate annealing (100–300 °C for 20 min) for two types of electrodeposited Mn oxide films with contrasting properties. Upon annealing, the originally inactive and structurally well-ordered Oxide 1 of birnessite type became as OER active as the non-heated Oxide 2, which has a highly disordered atomic structure. Oxide 2 also improved its activity upon heating, but more important is the stability improvement: the operation time increased by about two orders of magnitude (in 0.1 M KPi at pH 7). Aiming at atomistic understanding, electrochemical methods including quantitative analysis of impedance spectra, X-ray spectroscopy (XANES and EXAFS), and adapted optical spectroscopies (infrared, UV-vis and Raman) identified structure–reactivity relations. Oxide structures featuring both di-μ-oxo bridged Mn ions and (close to) linear mono-μ-oxo Mn3+–O–Mn4+ connectivity seem to be a prerequisite for OER activity. The latter motif likely stabilizes Mn3+ ions at higher potentials and promotes electron/hole hopping, a feature related to electrical conductivity and reflected in the strongly accelerated rates of Mn oxidation and O2 formation. Poor charge mobility, which may result from a low level of Mn3+ ions at high potentials, likely promotes inactivation after prolonged operation. Oxide structures related to the perovskite-like ζ-Mn2O3 were formed after the heating of Oxide 2 and could favour stabilization of Mn ions in oxidation states lower than +4. This rare phase was previously found only at high pressure (20 GPa) and temperature (1200 °C) and this is the first report where it was stable under ambient conditions.


 Please wait while we load your content...
Please wait while we load your content...