In situ formation of a multicomponent inorganic-rich SEI layer provides a fast charging and high specific energy Li-metal battery†
Abstract
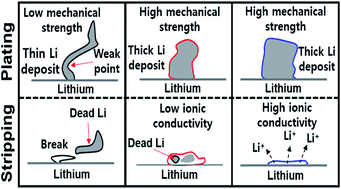
The performance of the rechargeable Li metal battery anode is limited by the poor ionic conductivity and poor mechanical properties of its solid-electrolyte interphase (SEI) layer. To overcome this, a 3 : 1 v/v ethyl methyl carbonate (EMC) : fluoroethylene carbonate (FEC) containing 0.8 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and 0.2 M lithium difluoro(oxalate)borate (LiDFOB) dual-salts with 0.05 M lithium hexafluorophosphate (LiPF6) was tested to promote the formation of a multitude of SEI-beneficial species. The resulting SEI layer was rich in LiF, Li2CO3, oligomeric and glass borates, Li3N, and Li2S, which enhanced its role as a protective yet Li+ conductive film, stabilizing the lithium metal anode and minimizing dead lithium build-up. With a stable SEI, a Li/Li[Ni0.59Co0.2Mn0.2Al0.01]O2 Li-metal battery (LMB) retains 75% of its 177 mA h g−1 specific discharge capacity for 500 hours at a coulombic efficiency of greater than 99.3% at the fast charge–discharge rate of 1.8 mA cm−2.


 Please wait while we load your content...
Please wait while we load your content...