Ambient electrohydrogenation of N2 for NH3 synthesis on non-metal boron phosphide nanoparticles: the critical role of P in boosting the catalytic activity†
Abstract
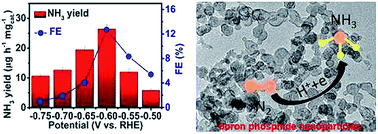
As a carbon-neutral alternative to the Haber–Bosch process, electrochemical N2 reduction enables environment-friendly NH3 synthesis under ambient conditions but needs active electrocatalysts for the N2 reduction reaction (NRR). In this communication, we report on the first experimental demonstration that non-metal boron phosphide (BP) nanoparticles can be used as a high-efficiency catalyst for the ambient electrohydrogenation of N2 to NH3 with excellent selectivity. In 0.1 M HCl, this catalyst offers a high NH3 yield of 26.42 μg h−1 mgcat.−1 and a high faradaic efficiency of 12.7% at −0.60 V vs. the reversible hydrogen electrode, much superior to those of reported B catalysts. Such an enhancement is attributed to the fact that P in BP further weakens the N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond while simultaneously strengthening the B–N bond and favors the exposure of more active sites for the NRR catalysis, which is supported by density functional theory calculations.
N bond while simultaneously strengthening the B–N bond and favors the exposure of more active sites for the NRR catalysis, which is supported by density functional theory calculations.
- This article is part of the themed collection: 2019 Journal of Materials Chemistry A Most Popular Articles


 Please wait while we load your content...
Please wait while we load your content...