Killing two birds with one stone: a Cu ion redox mediator for a non-aqueous Li–O2 battery†
Abstract
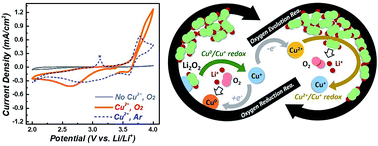
The aprotic Li–O2 battery is considered as a promising high-energy storage system although its practical application is limited by high overpotential and parasitic reactions. Herein, it is shown that by the addition of a soluble Cu ion, the electrochemistry of Li2O2 for both discharge and charge processes can be mediated by different redox couples of the Cu ion. The improved capacity was achieved by a Cu+/Cu0 redox-mediated reaction pathway, and the 3.6 V Cu+/Cu2+ redox couple helped reduce the charge overpotential. Moreover, because of the suppression of the reactive superoxide intermediate and a mild charge potential, the parasitic reaction was effectively inhibited. Not rigidly limited within the organic compound candidates, the introduction of the inorganic Cu ion, as a multi-functional mobile additive, would open up a new pathway for the design of metal-ion redox mediators for Li–O2 batteries.


 Please wait while we load your content...
Please wait while we load your content...